Abstract
Introduction
Acute graft-versus-host disease (aGVHD) is a significant cause of mortality post allogeneic stem cell transplantation (SCT). First line therapy is corticosteroid based for patients with aGVHD ≥grade (Gd) II. Steroid refractory (SR) aGVHD is associated with a dismal prognosis. Although experimental therapeutics is an unmet need, there is no recent prospective real world data to establish a benchmark for outcomes of best available therapy. We undertook a prospective observational multi-centre international clinical trial of outcomes of second line therapy of steroid refractory aGVHD. The aim was to measure recently defined endpoints including Day 28 (D28) overall response rate (ORR), and 6 month freedom from treatment failure (6mFFTF; defined as a patient (pt) being alive, without relapse of underlying disease or addition of new systemic therapy for aGVHD, prior to the development of chronic(c) GVHD.
Methods
Data was collected prospectively in a longitudinal observational study in patients starting second line therapy for SR aGVHD. Twelve UK and 2 US sites participated. Adult pts with Gd II-IV aGVHD refractory to corticosteroids were enrolled within 5 days of starting second line therapy. Modified Glucksberg criteria were used to grade aGVHD. Expert panel consensus recommendations (Martin et al 2009) were used for complete response (CR), very good partial response (VGPR), partial response (PR), stable disease (SD) and progressive disease (PD). Kaplan-Meier survival curves and log rank tests for comparison were done on Prism7.
Results
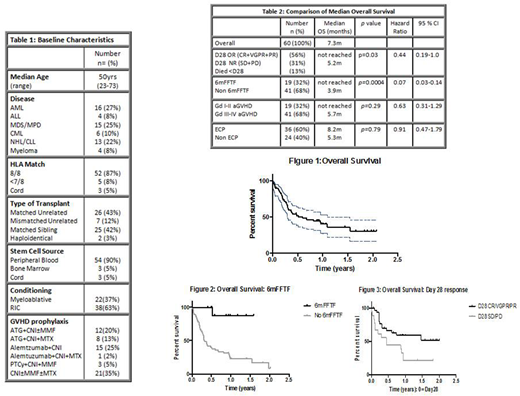
Between 2014-2018, 64pts were enrolled; 4pts were excluded due to incomplete data. Baseline characteristics of the cohort are outlined in Table 1. GVHD prophylaxis varied according to centre. Forty-seven percent had T depletion; 32% ATG and 25% Alemtuzumab. GVHD was donor lymphocyte infusion (DLI) induced in 7%.
At start of second line therapy,5% had Gd I, 27% Gd II and 68% Gd III-IV aGVHD. All patients had received corticosteroids at a minimum dose of 1mg/kg. Second line therapies varied, 60% received ECP and 40% non-ECP therapy including anti-TNF-alpha antibodies (20%), mycophenolate mofetil (MMF) (8%), mesenchymal stem cells (4%) and 'other' (8%).
The cumulative incidence of 6mFFTF was 32% (n=19), with 68% (n=41) failing to meet the 6mFFTF endpoint. The causes of failure were, addition of third line therapy in 40% (n=24), relapse 8%(n=5), cGVHD 7% (n=4) and death 13% (n=8). The 6m incidence of death was 45% (n=27); death due to GVHD 30%, infection 8% and relapse 7%. D28 responses were: 56% ORR (8% CR, 28% VGPR, 20% PR), 13% SD, 18% PD and 13% died before D28. Using Kaplan-Meier analysis, median OS was 7.3m with a median follow up of 1 year. The overall cumulative incidence of death was 63% (n=38); GVHD was the leading cause of death 40%, infection 8% and relapse 8%. D28 ORR was associated with a significantly improved median OS not reached versus (vs) 5.2m (p=0.03) in pts who had SD or PD at D28. In pts that achieved 6mFFTF, median survival beyond 6m was not yet reached vs median survival of 3.9m in those that did not achieve 6mFFTF (p=0.0004). The trend was for a longer median OS of 8.2m in the ECP arm vs 5.3m in non ECP (p=0.79). GVHD Gd at start of second line therapy was associated with a longer median survival, not reached in Gd II cohort vs 5.7m in Gd III-IV cohort (p=0.25).
Discussion
Our multi-centre prospective study illustrates 'real-life' data of clinical outcomes in pts starting second line therapy for SR aGVHD. Median survival from start of second line therapy was 7.3m with a high mortality rate of 63%. D28 ORR was 56% but only 32% met the composite 6mFFTF endpoint, although both outcomes predicted survival. The leading cause for failure of 6mFFTF was starting third line therapy which may be subject to clinician bias, however the 6m mortality was 45%, thus pts went on to die after failing for another cause. This data can be used as a contemporary data while planning studies of experimental intervention vs. best available therapy.
Radia:Mallinckrodt: Research Funding. Alfred:Mallinckrodt: Speakers Bureau. Dignan:Mallinckrodt: Research Funding, Speakers Bureau. Jagasia:Incyte Corporation: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal